
We write electron configuration according to neutral state of element. Thus, X has 12 electrons in neutral state. In neutral element, number of proton is equal to number of electrons. Ions and isotopes of elements are not shown in periodic table.Įxample: If X +2 ion has 10 electrons, find its group and period number.
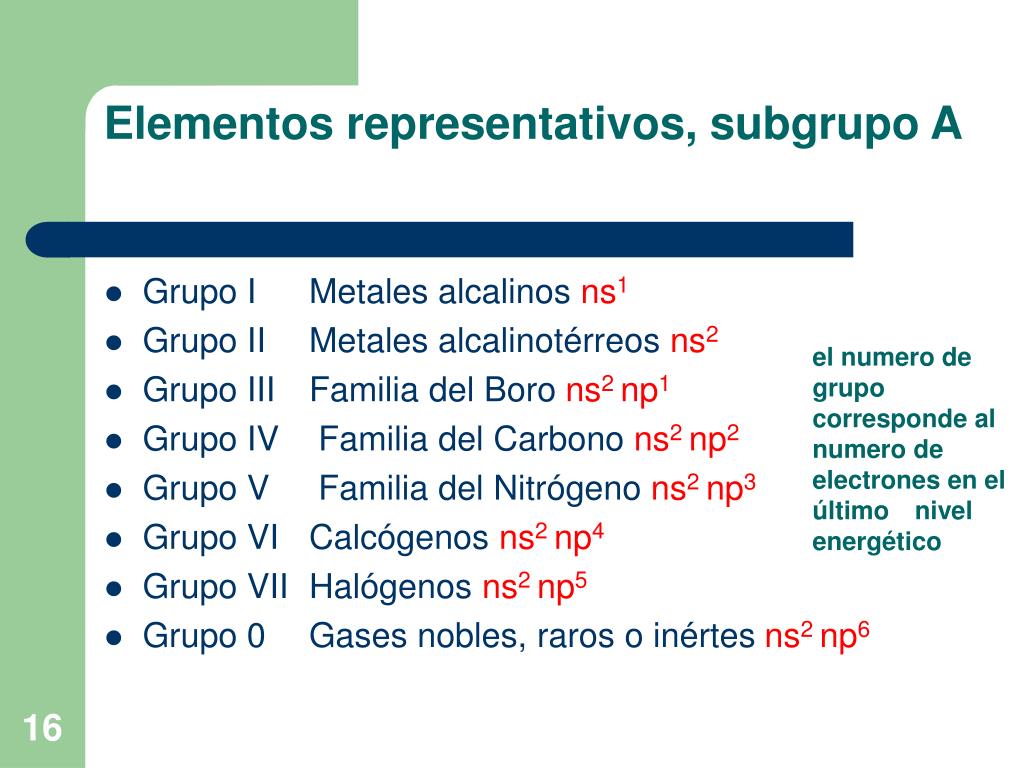
Groups and Periods of elements are found according to their neutral states. Here are some clues for you to find group number of elements. Look at following examples.Ģ6Fe: 1s 22s 22p 63s 23p 6 4s 23d 6 6+2=8 B group If last sub shell of electron configuration is "s" or "p", then group becomes A.ġ9K : 1s 22s 22p 63s 23p 64 s 1 Since last sub shell is "s" group of K is A.ģ5Br : 1s 22s 22p 63s 23p 64s 23d 104 p 5 Since last sub shell is "p" group of Br is A.Įlements in group B have electron configuration ns and (n-1)d, total number of electrons in these orbitals gives us group of element. Another way of finding group of element is looking at sub shells. Group of element is equal to number of valence electrons of element or number of electrons in the highest energy level of elements. Thus period of S is 3.Ģ3Cr: 1s 22s 22p 63s 23p 6 4s 23d 4 4 is the highest energy level of electrons or principal quantum number.
.jpg)

Look at following examples for better understanding ġ6S: 1s 22s 22p 6 3s 2 3p 4 3 is the highest energy level of electrons or principal quantum number. Period of the element is equal to highest energy level of electrons or principal quantum number. Finding Location of Elements in Periodic Table with Examples


 0 kommentar(er)
0 kommentar(er)
